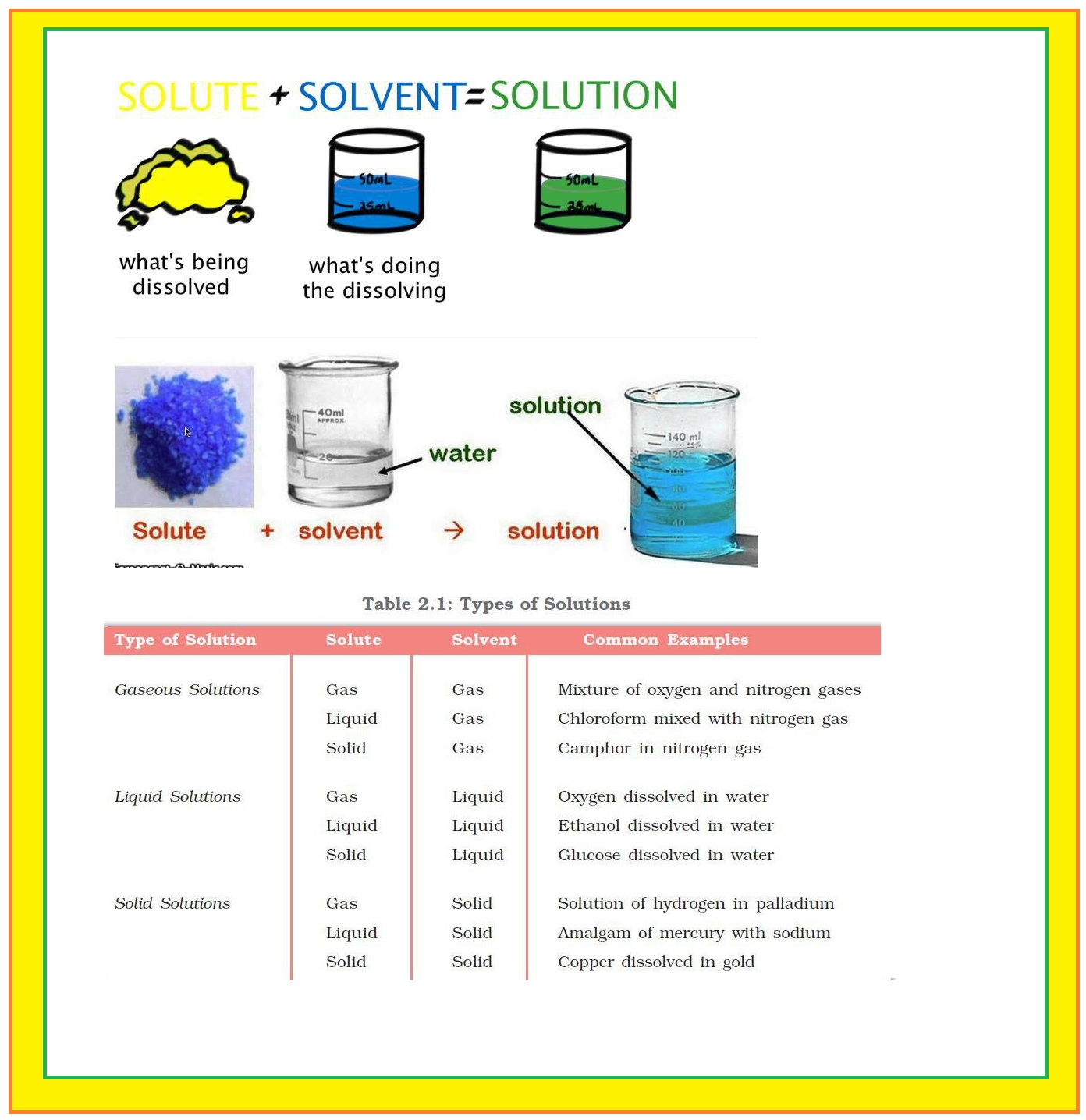
Types of Solutions :
`color{purple}(✓✓)color{purple} " DEFINITION ALERT"`
`color{green} ("Homogeneous Solutions")` : These are mixtures of two or more than two components. These have uniform composition and properties throughout the mixture.
`color{green}("Solvent")` : The component that is present in the largest quantity is known as solvent. Solvent determines the physical state of solution.
`color{green}("Solute")` : One or more components present in the solution other than solvent are called solutes.
`color{green}("Dilute solution")` : A solution in which relatively a small amount of solute is dissolved in large amount of solvent is called a dilute solution.
`color{green}("Concentrated solution")` : A solution in which relatively a large amount of the solute is present is called a concentrated solution.
`color{green}("Note") :` We shall consider only binary solutions (two component solutions) in this unit.
`color{green} ("Homogeneous Solutions")` : These are mixtures of two or more than two components. These have uniform composition and properties throughout the mixture.
`color{green}("Solvent")` : The component that is present in the largest quantity is known as solvent. Solvent determines the physical state of solution.
`color{green}("Solute")` : One or more components present in the solution other than solvent are called solutes.
`color{green}("Dilute solution")` : A solution in which relatively a small amount of solute is dissolved in large amount of solvent is called a dilute solution.
`color{green}("Concentrated solution")` : A solution in which relatively a large amount of the solute is present is called a concentrated solution.
`color{green}("Note") :` We shall consider only binary solutions (two component solutions) in this unit.





